The Preclinical Bottleneck
Traditional preclinical systems, like 2D cell lines or animal models, often fall short when evaluating new therapies. They can’t recapitulate the complexity of human tissues, immune interactions, or patient variability. This is especially problematic for cell therapies, biologics, or immune-targeting drugs, where outcomes depend heavily on dynamic, multicellular interactions.
Even newer tools like epithelial-only organoids, though more advanced than 2D cultures, remain reductionist. By isolating the epithelial compartment from stromal and immune influences, they miss critical microenvironmental signals that shape disease progression, therapeutic response, and tissue remodeling. Artificial co-culture methods attempt to reintroduce these elements, but they rarely mimic how such compartments self-organize in vivo. The result is a system that may contain the “right” cell types but lacks the emergent spatial structure and signaling dynamics necessary to model disease accurately.
Modern pipelines need more than simplified systems. They require predictive platforms that reflect:
• Tissue-specific architecture and signaling
• Disease heterogeneity across patients
• Dynamic microenvironment interactions including immune responses

Getting Closer to Real Patient Biology
Advanced preclinical platforms that mimic native tissue complexity, especially those that inherently include epithelial, stromal, and immune compartments are reshaping how biopharma evaluates new therapies. These models are more than just tools for testing—they provide insight into disease mechanisms, stratify patient responses, and reduce the risks of clinical failure.
Four Levers That Matter in Preclinical Strategy
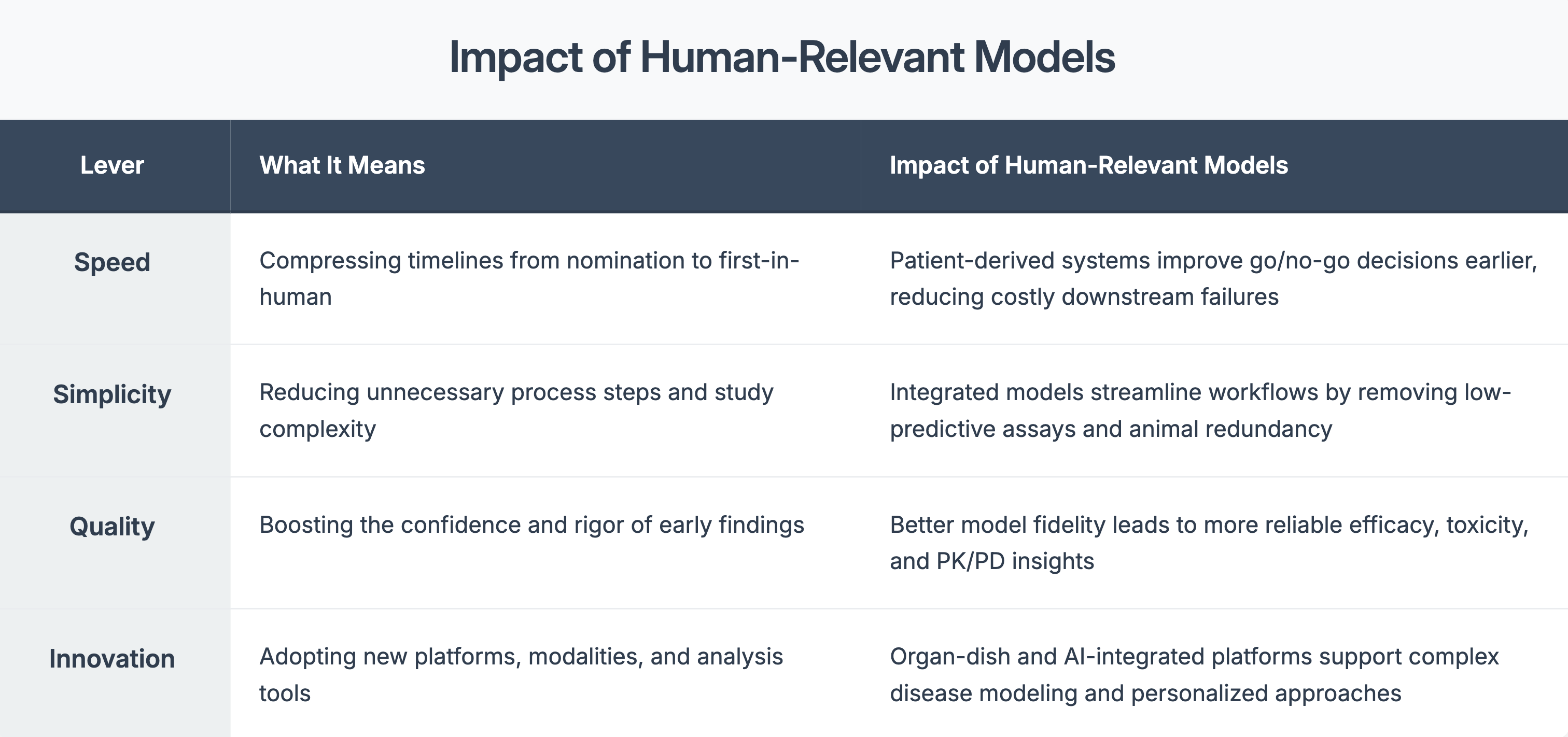
When used early, they can:
• Flag toxicity profile before trials begin
• Reveal differences in drug response across patient subtypes
• Guide dose optimization with more confidence
- Support regulatory filings with more compelling data
References
- 1. Agrawal G, Bader F, Günthner J, Wurzer S. Fast to first-in-human: Getting new medicines to patients more quickly. McKinsey & Company; 2023. 2. Harvard Wyss Institute. Beyond animals: Revolutionizing drug discovery with human-relevant models. 2024. 3. Charles River. Leveraging advanced technology to expedite oncology R&D. 2024. 4. Regulatory Scoping Review. Clinical relevance in preclinical model selection. PharmTech & PubMed; 2024. 5. Frontiers in Toxicology. AI-powered digital toxicology platforms. 2024. 6. Wikipedia. 3D Cell Culture & Patient-Derived Xenografts. Accessed 2024.